Laboratory of Pollen Biology
of the Institute of Experimental Botany, Czech Academy of Sciences
studies plant reproductive development, namely the regulatory mechanisms at different levels:

1. Transcriptional Regulation during Male Gametophyte Development
2. Translational Regulation and mRNA Storage in Pollen and Pollen Tubes
3. Male-Female Dialogue between Reproduction Partners Preceding Double Fertilisation
Research Activity
The Laboratory of Pollen Biology was founded in the 1990s and has since been continuously engaged in exploring fundamental aspects of plant reproductive biology, with a particular focus on sexual reproduction and genome stability. Over the years, our work has helped shape the field, particularly through early studies in pollen developmental transcriptomics (Honys and Twell 2003; 2004). These efforts yielded pioneering insights, including one of the first effective examples of gene expression dynamics in individual plant cells, which contributed to the current gene-centric approaches in male gametophyte research.
Our research targets key stages and mechanisms of pollen development, including its resilience to heat stress, communication with female reproductive tissues, genomic maintenance, and responses to environmental signals. We apply a comprehensive methodological framework combining molecular and cell biology, genetics, proteomics, transcriptomics, and phylogenetics. In response to current global challenges, such as climate change, we are increasingly interested in developing experimental strategies to manipulate gametophyte development and function for potential applications in plant breeding. While Arabidopsis thaliana and Nicotiana tabacum remain our primary model species, we also conduct research on tomato, pear, Amborella, Physcomitrium, and seaweed, often within collaborative frameworks.
Our work is organized into four interconnected research areas:
1. Molecular Mechanisms of Male Gametophyte Development and Function
This core research area aims to understand the molecular mechanisms governing male gametophyte (pollen) development, maturation, and its crucial role in successful plant reproduction (Hafidh et al. 2016; Hafidh and Honys 2021).
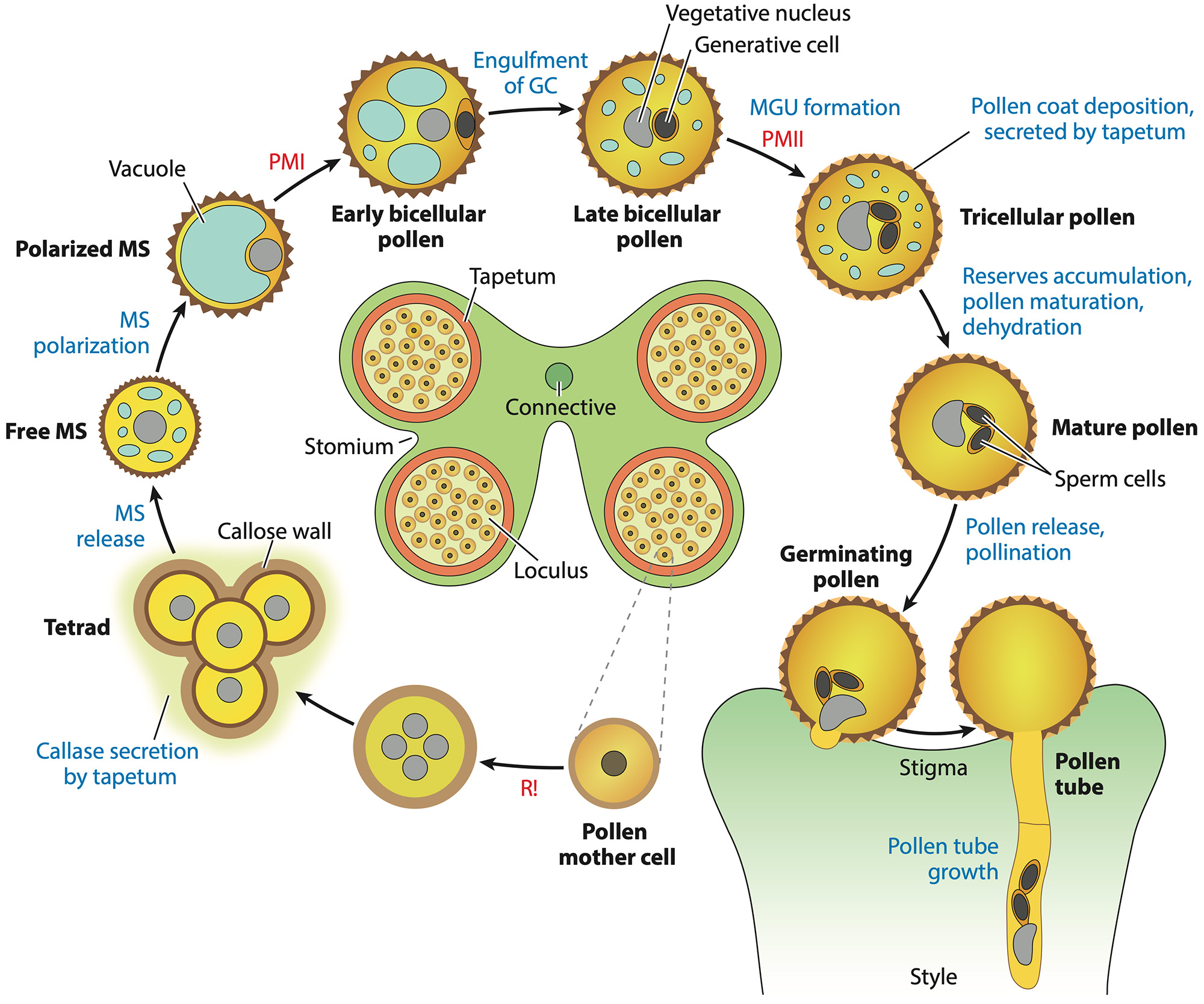
Pollen development (Hafidh and Honys 2021).
1.1. Genetic and Post-Transcriptional Regulation of Pollen Development
A significant part of our work involves identifying and characterizing important genetic factors and regulatory pathways. We focus on bZIP family transcription factors and their role in pollen stress response (Gibalová et al. 2009; 2017; Wiese et al. 2021). Our collaborative work on the microspore-specific GAMYB transcription factor MYB81 (Oh et al. 2020) helped defining its role in promoting pollen mitosis I and cell lineage formation in Arabidopsis. This discovery highlights an important regulatory switch in male gametophyte development.
We also conduct 'omics' work on post-transcriptional regulation (Hafidh et al. 2018; Klodová et al. 2023), including its relation to pollen stress response. For example, we demonstrated the critical role of LARP6C in regulating selective mRNA translation to promote pollen tube guidance in Arabidopsis (Biley et al. 2021). This protein orchestrates the timely reprogramming of gene expression during the transition from quiescent pollen to active pollen tubes, underscoring the importance of post-transcriptional control in plant fertilization. We have characterized the eukaryotic translation initiation factor eIF3 (Raabe et al. 2019) and its subunits, showing that eIF3M2 is important for maintaining pollen tube integrity during heat stress (Kahrizi et al. 2025). Furthermore, ALBA family proteins (Náprstková et al. 2021) show dynamic expression and localization in Arabidopsis generative organs, particularly in mature pollen, where they are involved in RNA metabolism and stress response. Similarly, the Nascent Polypeptide Associated Complex (NAC) beta subunit is important for flower and silique development, with implications for male gametophyte development and stress responses (Fíla et al. 2020).
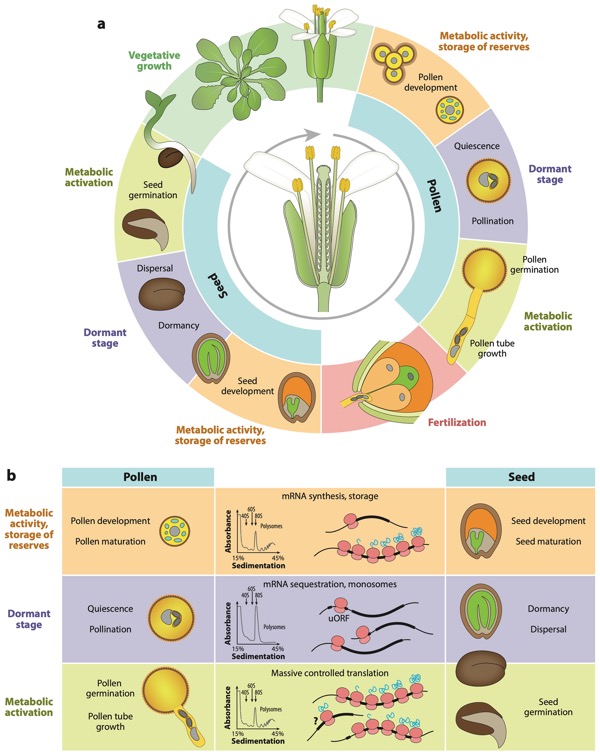
Translational and posttranslational regulation in the male gametophyte, including the scheme and players involved. (a) Simplified angiosperm life cycle highlighting the similar phases of pollen and seed development, including development, quiescence/dormancy, and activation, that are reflected in the accumulation, storage,and activation of mRNA reserves. (b) Our transcriptome and proteome analyses revealed that pollen and the pollen tube sequestrome comprised diverse mRNA populations that are highly dynamic between the actively translating polysomes and the sequestered monosome subfractions. Indeed, the monitoring of selected mRNAs encoding pollen tube cell wall proteins demonstrated a progressive shift in the majority of these transcripts from the monosomes to polysome fractions upon pollen activation. mRNA storage, activation, and translation, in general, are facilitated by numerous RBPs; this is also the case in the male gametophyte and in seeds. RBPs often bind cis-elements of the regulated mRNA sequences, namely their 5′ UTRs, including uORFs. Interestingly, highly similar sequence motifs were identified in 5′ UTRs of monosome-associated stored transcripts in pollen and seeds. Therefore, in pollen tubes and perhaps in other tip-growing cell types, the mRNA-bound monosomes likely represent the sequestrome pool of stored mRNAs. The question mark denotes hypothetical translational activation of a specific subtype of monosome-bound mRNA. Abbreviations: mRNA, messenger RNA; RBP, RNA-binding protein; uORF, upstream open reading frame; UTR, untranslated region (Hafidh and Honys 2021).
1.2. Pollen Tube Guidance and Secretome Dynamics
Precise guidance of pollen tubes to the ovule is essential for fertilization. Our research found that PRP8A and PRP8B spliceosome subunits regulate pollen tube attraction in Arabidopsis (Kulichová et al. 2020). These paralogs are vital for both ovule attraction and pollen tube sensing of attraction signals, linking pre-mRNA splicing to cell-to-cell communication during reproduction. This complements our work on the pollen tube secretome (Hafidh et al. 2016) and the development of a robust protocol for isolating pistil-stimulated pollen tube secreted proteins (Hafidh and Honys 2020). This methodological advancement allows for in-depth proteome coverage and has been instrumental in comparative analysis of basal angiosperm Amborella secretome (Flores Tornero et al. 2021). We helped to identify conserved and species-specific secreted proteins crucial for cell wall processes, signalling, and metabolism, providing a valuable resource for functional studies.
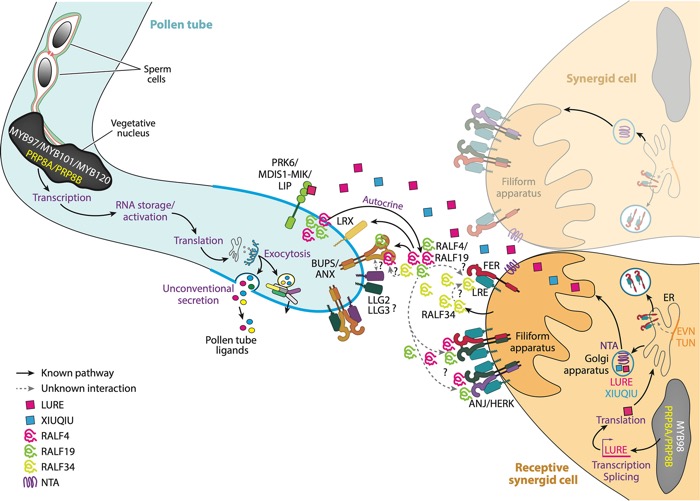
Pollen tube ovular guidance and attraction by the receptive synergid cell (Hafidh and Honys 2021).
1.3. Pollen Physiology and Stress Responses
We investigate how pollen responds to various environmental cues, particularly heat stress, as summarized in Chaturvedi et al. 2021.
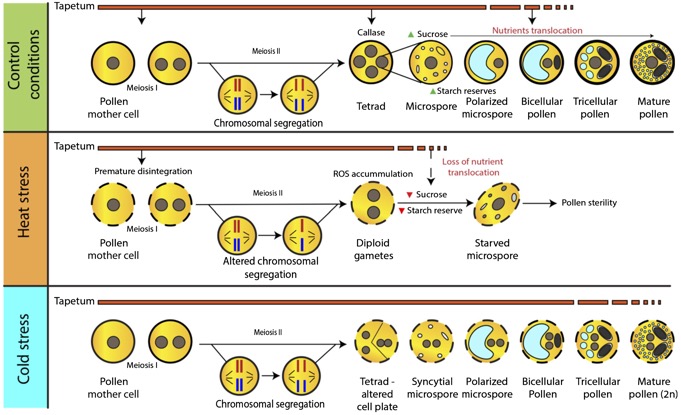
Cytological alterations caused by heat and cold stress during pollen development (Chaturvedi et al. 2021).
Our experimental work demonstrated that Arabidopsis bZIP18 and bZIP52 transcription factors accumulate in nuclei following heat stress, jointly regulating gene expression and metabolic pathways (Wiese et al. 2021). Our recent findings further emphasize the importance of specific proteins in maintaining pollen tube integrity under heat stress. The eIF3M2 protein was shown to maintain pollen tube integrity during heat shock via HSP70 upregulation (Kahrizi et al. 2025). This critical discovery identifies a novel eIF3M2/HSP70 regulatory module for thermotolerance adaptation in plants. Moreover, Arabidopsis pollen heat stress resilience is conveyed also by HSP90 (Kahrizi et al. 2025). Our international team also provided a detailed analysis of transcript and protein accumulation waves accompanying pollen dehydration (Sze et al. 2024), revealing processes that protect pollen from hyperosmotic stress and equip it for rehydration, a crucial aspect for successful fertilization.
1.4. Evolution of Reproductive Mechanisms
We contribute to large-scale comparative analyses to understand the evolution of plant reproduction. A significant output is the comparative transcriptomic analysis revealing conserved programs underpinning organogenesis and reproduction across diverse land plants, from bryophytes to flowering plants (Julca et al. 2021). This extensive collaborative effort identified hundreds of organ- and gamete-specific orthogroups and suggested that gene co-option is a main mechanism for evolving new organs. The study specifically highlighted the high specialization and conservation of male reproduction. Similarly, we were part of another large international consortium that defined the peri-germ membrane surrounding pollen sperm cells as an integral and important component of the male germ unit in angiosperms (Sugi et al. 2024), playing a critical role in sperm cell delivery and double fertilization. Finally, we reviewed recent progress in molecular mechanisms involved in plant reproduction, the evolution of heterospory, timing of pollen mitosis II (the distribution of bicellular and tricellular pollen within angiosoperms), and the importance of post-transcriptional regulation and cell-cell communication in this process (Hafidh and Honys 2021).
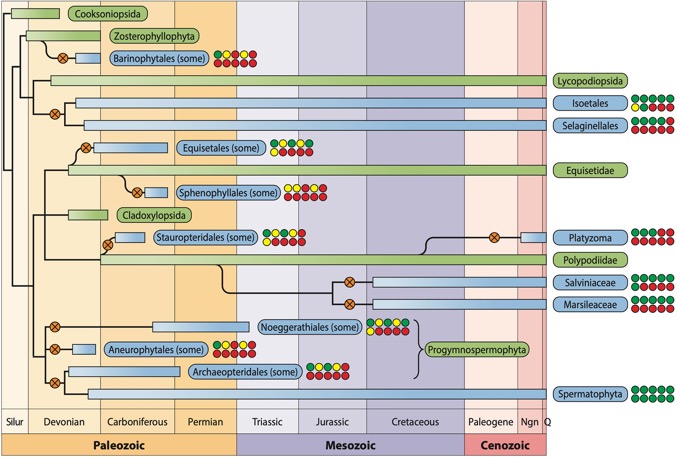
Evolution of heterospory among land plants. Simplified phylogenetic tree showing 11 documented heterosporous lineages of vascular plants. Green rectangles represent the main lineages, predominantly isosporous, from which heterosporous lineages evolved. Blue rectangles stand for heterosporous plant lineages. Rounded black branches show the evolution of side lineages when the exact timing is not always precisely resolved. Orange circles with crosses depict the 11 events related to the appearance of heterospory in individual lineages; they phylogenetically represent the evolutionary events, not their timing. Ten circles show whether each of ten selected phenomena associated with heterospory has been identified within individual plant lineages (green, yes; yellow, probably yes/uncertain; red, no).These phenomena are sequentially: (upper row) heterospory, dioecy, heterosporangy, endospory, monomegaspory, (lower row) endomegasporangy, integumentation, in situ pollination, in situ fertilization, and PT formation. Abbreviations: Ngn, Neogene; Q, Quarternary; Silur, Silurian (Hafidh and Honys 2021).
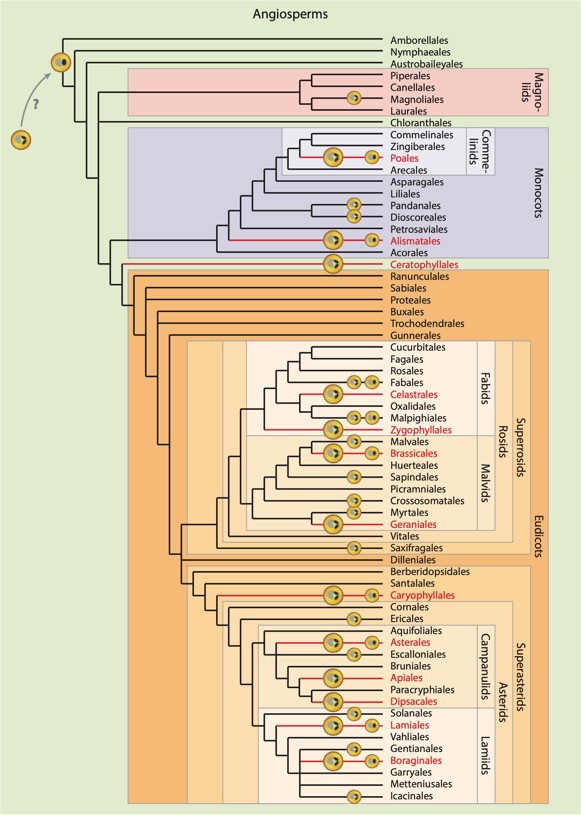
Distribution of tricellular and bicellular pollen within angiosperms. Tricellular pollen is dominant in 13 orders (red lines and large tricellular pollen symbol, left pollen column). Several other orders also produce tricellular pollen (2–44% of species), but the majority are bicellular (small tricellular pollen symbol, middle pollen column). In nine orders, bicellular pollen evolved secondarily from tricellular ancestors (small bicellular pollen symbols, right pollen column). The possible tricellular ancestry of angiosperm pollen is reflected by the question mark in the top left corner.The mature pollen grain has a dehydrated cytoplasm, and bicellular pollen is usually shed in a more dehydrated and quiescent state than tricellular pollen. The simultaneous presence of both pollen types in one species is very uncommon and more likely in basal angiosperms. For instance, the coexistence of bi- and tricellular pollen grains in the same anther at the same time was observed in early-divergent angiosperm Annona cherimola. In A. cherimola, the production of the actual pollen type depends on environmental factors such as temperature and humidity during pollen maturation (Hafidh and Honys 2021).
1.5. Methodological Advancements in Pollen Research
Beyond fundamental discoveries, we actively develop and refine methodologies. The protocol for in vivo RNA labelling and visualization in tobacco pollen (Kumar et al. 2024) tubes provides a powerful tool for studying RNA-protein complexes in real-time. Additionally, the implementation of the Ribo-BiFC method to plant systems using a split mVenus approach (Raabe et al. 2024) offers a novel in vivo translation marker, enabling visualization of translational rates in plant tissues, which is vital for understanding dynamic gene expression during development and stress. We also contributed to the development of GOLEM, a tool for visualizing the distribution of gene regulatory elements within plant promoters (Nevosád et al. 2025), with a specific focus on the male gametophyte. This openly available tool is a significant contribution to the plant research community, facilitating the analysis of gene expression regulation across various plant species.
2. Genome Stability, DNA Repair, and Chromatin Dynamics
A distinct yet interconnected research theme focuses on the fundamental processes of genome maintenance, DNA repair, and chromatin organization, primarily utilizing the moss Physcomitrium patens as a model system due to its unique characteristics for genetic studies.
2.1. DNA Damage Response and Repair Mechanisms
We have made significant contributions to understanding how plants respond to and repair DNA damage. Our collaborative research demonstrated that DNA damage triggers reprogramming of differentiated cells into stem cells in Physcomitrium (Gu et al. 2020). This counterintuitive finding highlights a novel adaptive mechanism. Key components of the DNA repair machinery have been further characterized. The Kleisin NSE4 subunit of the SMC5/6 complex was found to be necessary for DNA double-strand break repair but not for recovery from DNA damage (Holá et al. 2021). This was further elaborated by showing that the NSE5 subunit interacts with distant regions of the SMC arms, suggesting its role in SMC5/6 loading to DNA lesions (Vaculíková et al. 2024). The roles of RAD51 and RAD51B in double-strand break repair in Physcomitrium were also elucidated, revealing diverse functions for these homologous recombination proteins (Angelis et al. 2023).
2.2. Chromatin Organization and Telomere Biology
In addition to studying DNA repair mechanisms, we also contribute to exploring the broader context of genome organization, with a particular focus on the structure and function of telomeres at chromosome ends. Within the field of telomere biology, we have contributed to advancing the understanding of the evolution of plant telomerase RNAs (Fajkus et al. 2021), tracing their deep conservation across diverse eukaryotic lineages. This research, led by the CEITEC Masaryk University and the Institute of Biophysics of the of the Czech Academy of Sciences, established a novel strategy for identifying telomerase RNAs and confirmed the templating role of a novel candidate in Physcomitrium. More recently, we contributed to the completion of the characterization of the TRB family of telomere repeat binding proteins, revealing their ancient evolutionary origins, distinct localization patterns, and conserved interactions with key protein complexes like telomerase and Polycomb repressive complex 2 (Kusová et al. 2023; 2025).
3. Plant Hormones, Metabolism, and Viroid-Host Interactions
This line of research investigates the role of endogenous plant hormones in developmental processes—particularly pollen development—as well as the intricate interactions between plants and viroid pathogens, including mechanisms of viroid elimination. While not a central focus, we have nonetheless contributed to several noteworthy findings in this area.
3.1. Hormonal Regulation of Plant Development
Our detailed study on hormonome dynamics during microgametogenesis in several Nicotiana species provided a comprehensive analysis of phytohormone profiles throughout pollen ontogenesis (Záveská Drábková et al. 2021). This work identified the involvement of specific hormone forms in pollen development and highlighted phylogenetic differences in hormone accumulation. Furthermore, we investigated the evolutionary diversification of cytokinin-specific glucosyltransferases in angiosperms, shedding light on the enigma of missing cis-zeatin O-glucosyltransferase genes in Brassicaceae (Záveská Drábková et al. 2021).
3.2. Plant-Viroid Interactions and Elimination
We have contributed to the pioneering research on viroid elimination from pollen. Early work demonstrated that the elimination of viroids from tobacco pollen involves a decrease in propagation rate and an increase in degradation processes (Matoušek et al. 2020). This was further supported by integrated proteo-transcriptomic analyses, which provided mechanistic insights into viroid eradication during the final stages of pollen development in Nicotiana tabacum (Shrestha et al. 2020). Further research explored the impact of "forcing" overexpression of seed non-transmissible hop viroids in Nicotiana benthamiana, showing that this leads to strong pollen pathogenesis and developmental distortions (Steinbachová et al. 2021). These findings suggest that viroid adaptation to pollen metabolism is a crucial factor for their transmissibility.
4. Enhancing Crop Reproductive Resilience to Abiotic Stress
A growing focus of our research addresses climate change impacts on plant reproduction and aims to develop strategies for improving crop resilience in the context of global food security, marking our shift toward applied research and the translational potential of our results.
We actively participate in initiatives addressing the global challenge of climate change. A recent review, involving a large international consortium, discussed integrative approaches to enhance reproductive resilience of crops for climate-proof agriculture (Agho et al. 2025; Chopra et al. 2025). This comprehensive paper provides an overview of how abiotic stresses related to climate change affect plant reproduction and discusses the collective contribution of genetics, breeding technologies, biotechnological innovations, and sustainable agronomic practices. It also highlights the potential of Artificial Intelligence (AI) in optimizing breeding strategies. It is a direct output of the RECROP COST Action (CA22157), a significant European collaborative initiative aimed at transforming efforts for improving crop resilience.
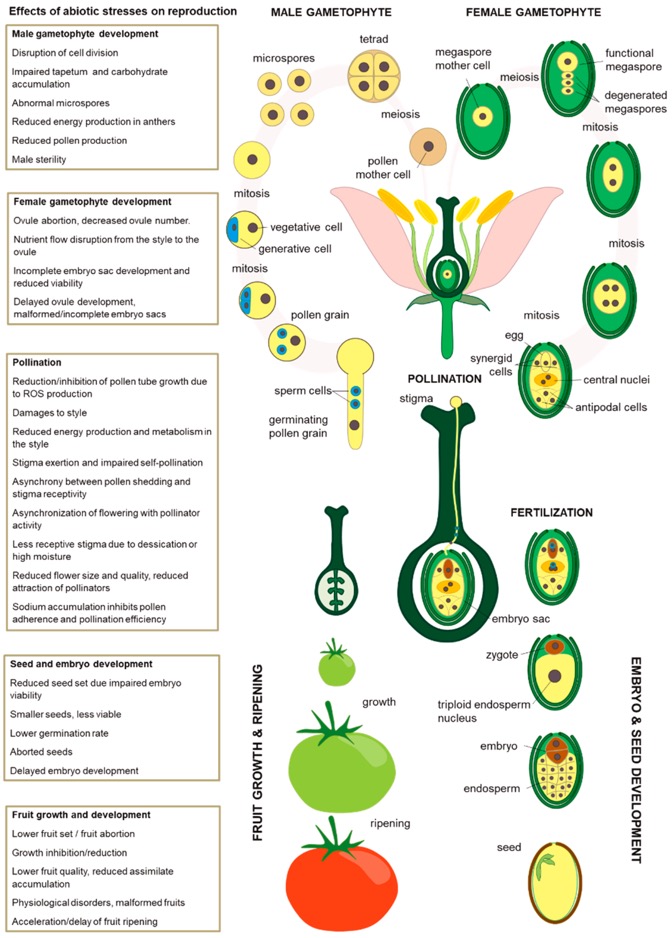
Impact of abiotic stresses on plant sexual reproduction (Agho et al. 2025).
While much of our research is fundamental, the implications for crop improvement are consistently highlighted. We have identified several promising candidate genes and proteins for validation and further exploitation for breeding to develop heat-tolerant crop varieties. In collaboration with Ghent University, we addressed potential issues related to T-DNA-associated mutagenesis (Raabe et al. 2024), providing guidelines for evaluating T-DNA-mutagenized transgenic lines and reducing the risk of misinterpretation in fertility and gametogenesis analyses.
Doctoral / Postdoctoral position in Pollen Biology
We are continuously seeking for a talented and highly motivated candidate with excellent communications skills and the ability to work in a team on an interdisciplinary project. For postdoc position, PhD degree in natural sciences/life sciences (Plant Biology, Biochemistry, Microbiology, or Molecular Biology). Good knowledge in standard techniques of plant biology, cell biology, biochemistry and molecular biology are expected as well advanced bioinformatic skills. Experience in guidance of master and/or PhD students are assets.
For more information, please contact the group leader.